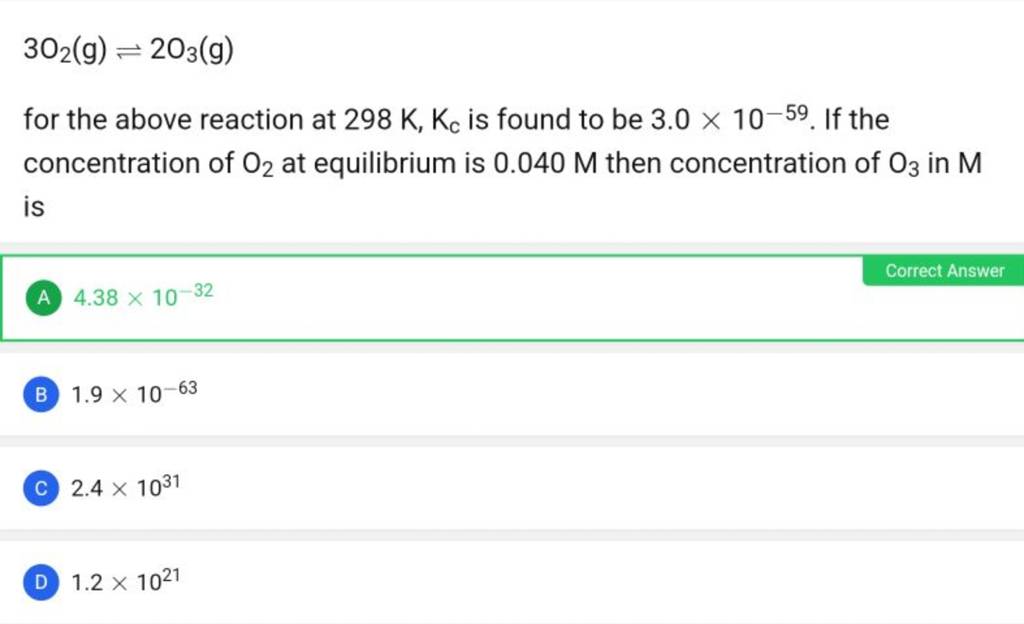
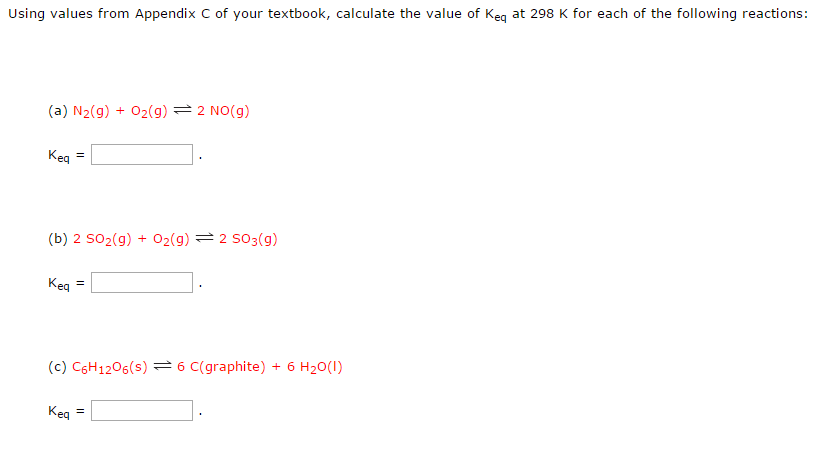
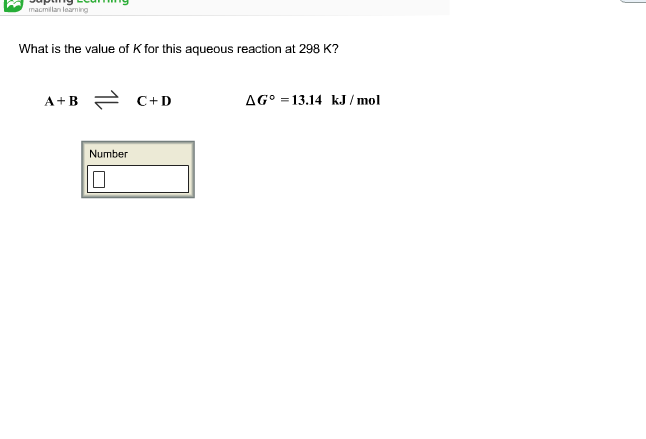
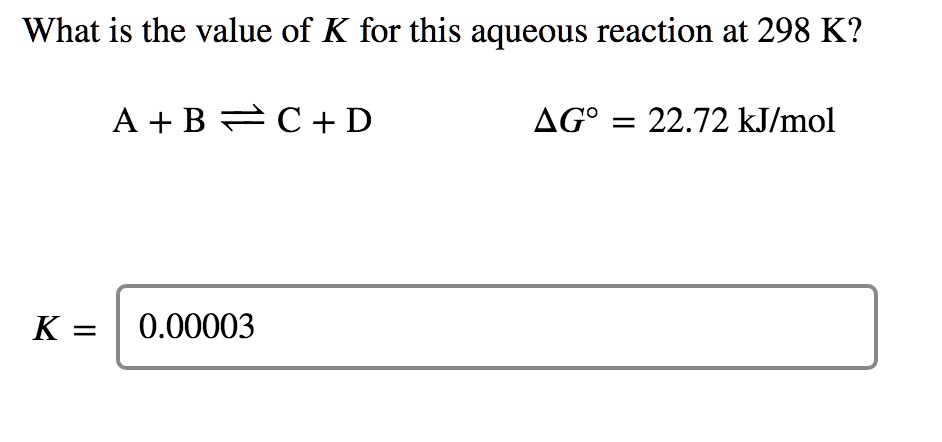Pls solve this: (ans - c) Q If at 298 K the bond energies of C H, - Chemistry - Chemical Kinetics - 12551821 | Meritnation.com

The Delta H^(0) for the following reaction at 298 K is -36.4 kJ 1//2 H_(2) (g) + 1//2Br_(2) (I) ... - YouTube
UV/Vis (CH2Cl2, l = 0.1 cm, 298 K) spectra overlay of a solution of 5... | Download Scientific Diagram
6) G (kJ/mol) values at 298 K for Ag+ (aq), Cl (aq) and AgCI (s) are 77, 129 and 109 respectively for the given reaction, Ag (aq) + C (aq) AgCI(s) Then

Dimensional Analysis. When you convert a given result from one system of units to another Method called unit factor method, or dimensional analysis. - ppt download